Abstract
BACKGROUND
LEN-based Tx is now a standard of care for patients with newly diagnosed multiple myeloma. Recently reported phase 3 trials conducted in patients with RRMM included only limited numbers of patients with LEN-based Tx failure immediately prior to study entry. By design, the MM-014 (NCT01946477) trial included patients with first- or second-line LEN-based Tx failures immediately prior to study entry. Cohort A of the MM-014 trial demonstrated that POM + LoDEX is safe and effective in this patient population.
Both POM and DARA have immunomodulatory effects, which when combined, may be additive or synergistic. The POM + LoDEX + DARA combination has demonstrated safety and efficacy in patients with RRMM. This combination was recently approved by the US Food and Drug Administration in the RRMM setting based on a trial conducted in heavily pretreated patients (median, 4 prior lines). However, the reported data did not indicate Tx received immediately prior to study entry.
To further assess the safety and efficacy of POM + LoDEX + DARA earlier in the Tx sequence (after LEN-based Tx failure in first or second line), a second cohort (B) was added to the MM-014 study. Initial results are reported here. The MM-014 trial also includes extensive biomarker correlatives to explore mechanisms of response and resistance to POM + LoDEX + DARA, which will be reported separately.
METHODS
Patients with documented RRMM after 1 or 2 prior lines who received prior Tx with a LEN-based regimen as their most recent Tx regimen and had progressive disease were eligible. A total of ≈ 100 patients are planned to be enrolled. In 28-day cycles, patients received POM 4 mg/day on days 1 through 21 + LoDEX 40 mg/day (20 mg/day if aged > 75 years) on days 1, 8, 15, and 22, and DARA 16 mg/kg intravenously on DEX dosing days of cycles 1 and 2, then days 1 and 15 of cycles 3 through 6, then day 1 of cycle 7 and beyond. Thromboprophylaxis was mandatory. The primary objective for cohort B is overall response rate (ORR) of POM + LoDEX + DARA based on modified International Myeloma Working Group criteria. Key secondary endpoints included adverse events (AEs) and second primary malignancies.
RESULTS
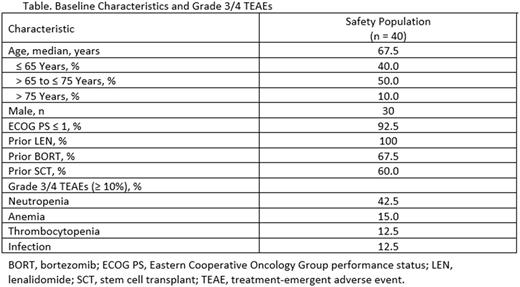
By the data cutoff date of May 3, 2017, 40 patients were enrolled. Median follow-up time was 1.6 months. Of 8 patients who discontinued Tx, 2 discontinued due to AEs, 2 due to progressive disease, and 4 due to other reasons. In the safety population (n = 40), median age was 67.5 years, 75.0% of patients were male, and 92.5% had an Eastern Cooperative Oncology Group performance status of ≤ 1 (Table). Of 35 patients with cytogenetic data by fluorescence in situ hybridization, 2, 4, and 4 patients were positive for del17p, t(4;14), and t(14;16), respectively. All patients received prior LEN, and 27 patients (67.5%) received prior bortezomib (BORT). Prior stem cell transplant was received by 24 patients (60.0%). Patients were either refractory to (70.0% [n = 28]) or had relapsed after (30.0% [n = 12]) LEN-containing Tx. Median duration of prior LEN-containing Tx was 23.7 months, with 45.0% of patients receiving 25 mg/day of LEN during their last LEN-containing regimen.
Grade 3/4 treatment-emergent AEs (≥ 10%; Table) included neutropenia (42.5%), anemia (15.0%), thrombocytopenia (12.5%), and infections (12.5%). There was 1 grade 3/4 pulmonary embolism and no reported grade 3/4 peripheral neuropathy. Infusion-related reactions of any grade were reported in 11 patients (27.5%). POM dose reduction due to neutropenia occurred in 4 patients (10.0%). POM dose interruptions due to AEs occurred in 14 patients (35.0%) and DARA dose interruptions due to AEs occurred in 21 patients (52.5%). Nine patients (22.5%) had POM dose interruptions due to neutropenia and 9 patients (22.5%) had DARA dose interruptions due to neutropenia. The mean relative dose intensity with POM and DARA was 0.9 and 0.7, respectively. In patients evaluable for efficacy (n = 25), the investigator-reported ORR was 68.0%.
CONCLUSIONS
The MM-014 trial (cohort B) demonstrated that Tx with POM + LoDEX + DARA was well tolerated and effective when sequenced as second or third line immediately after LEN-based Tx failure in patients with RRMM. In the context of a short follow-up time of 1.6 months, response and safety data compared favorably with the results of other doublet (DARA + DEX or POM + DEX) or triplet (POM + cyclophosphamide + prednisone or POM + BORT + DEX) regimens. Updated safety and efficacy data will be presented.
Siegel: Merck: Consultancy; Celgene, Takeda, Amgen Inc, Novartis and BMS: Consultancy, Speakers Bureau. Schiller: bluebird bio: Research Funding; mateon therapeutics: Research Funding. Sebag: Celgene, Janssen: Consultancy. Berdeja: Abbvie: Research Funding; Vivolux: Research Funding; Takeda: Research Funding; Teva: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Curis: Research Funding; Constellation: Research Funding; Celgene: Research Funding; BMS: Research Funding; Bluebird: Research Funding; Amgen: Research Funding. Ganguly: Amgen: Other: Advisory Board; Seattle Genetics: Speakers Bureau. Matous: Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Talamo: Penn State Hershey Cancer Institute: Employment. Bar: Celgene Corporation: Consultancy. Fonseca: Celgene Corporation: Research Funding, Speakers Bureau. Reece: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Mouro: Celgene Corporation: Employment, Equity Ownership. Agarwal: Celgene Corporation: Employment. Zafar: Celgene Corporation: Employment. Qian: Celgene Corporation: Employment, Equity Ownership. Thakurta: Celgene Corporation: Employment, Equity Ownership. Bahlis: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal